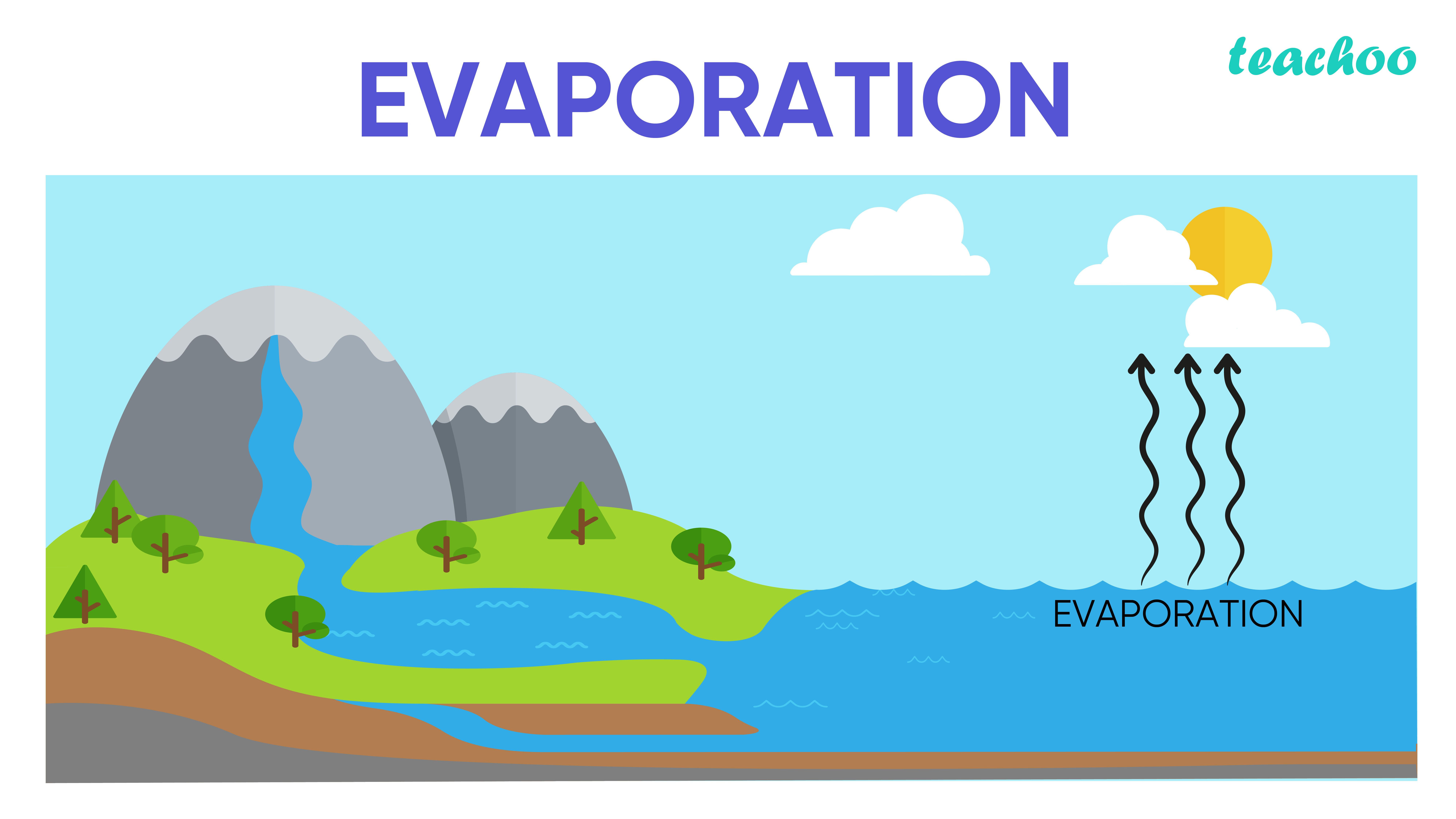
First Class Info About How To Increase Rate Of Evaporation

Multiply by the surface area of the water.
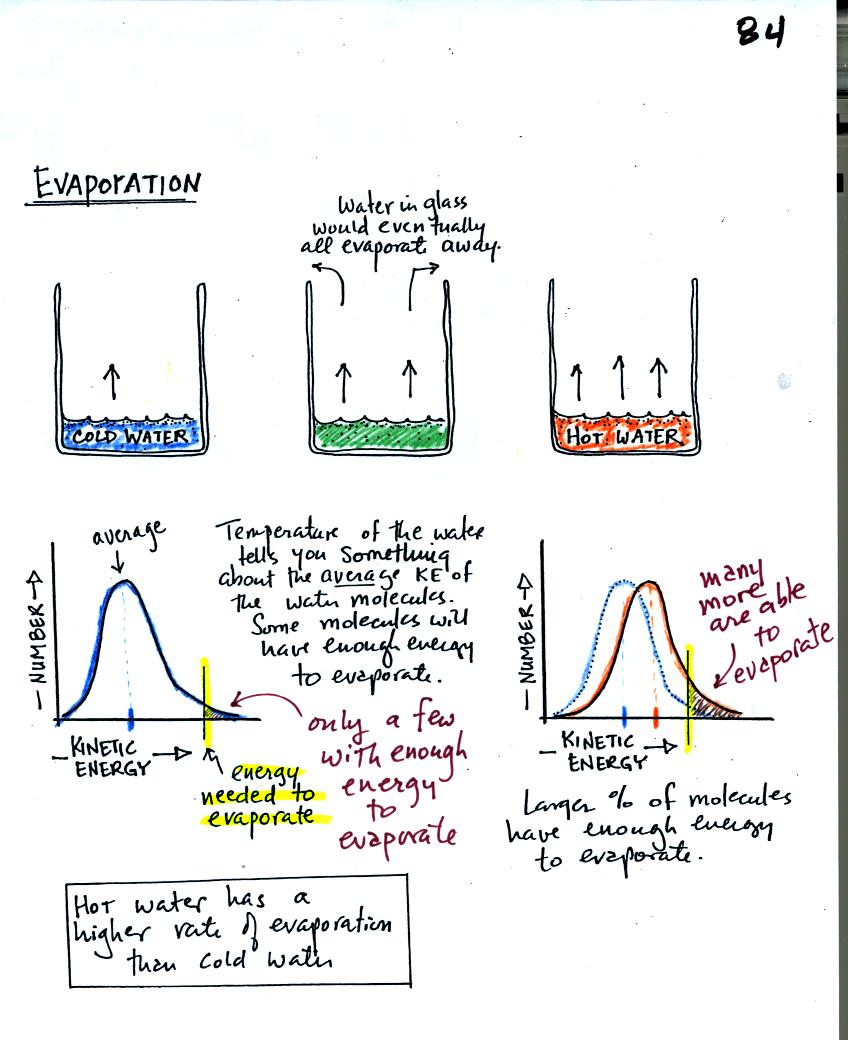
How to increase rate of evaporation. Increasing the temperature will increase the evaporation rate. But, with increased evaporation, more water molecules. Textbook solutions cbse notes what is evaporation?
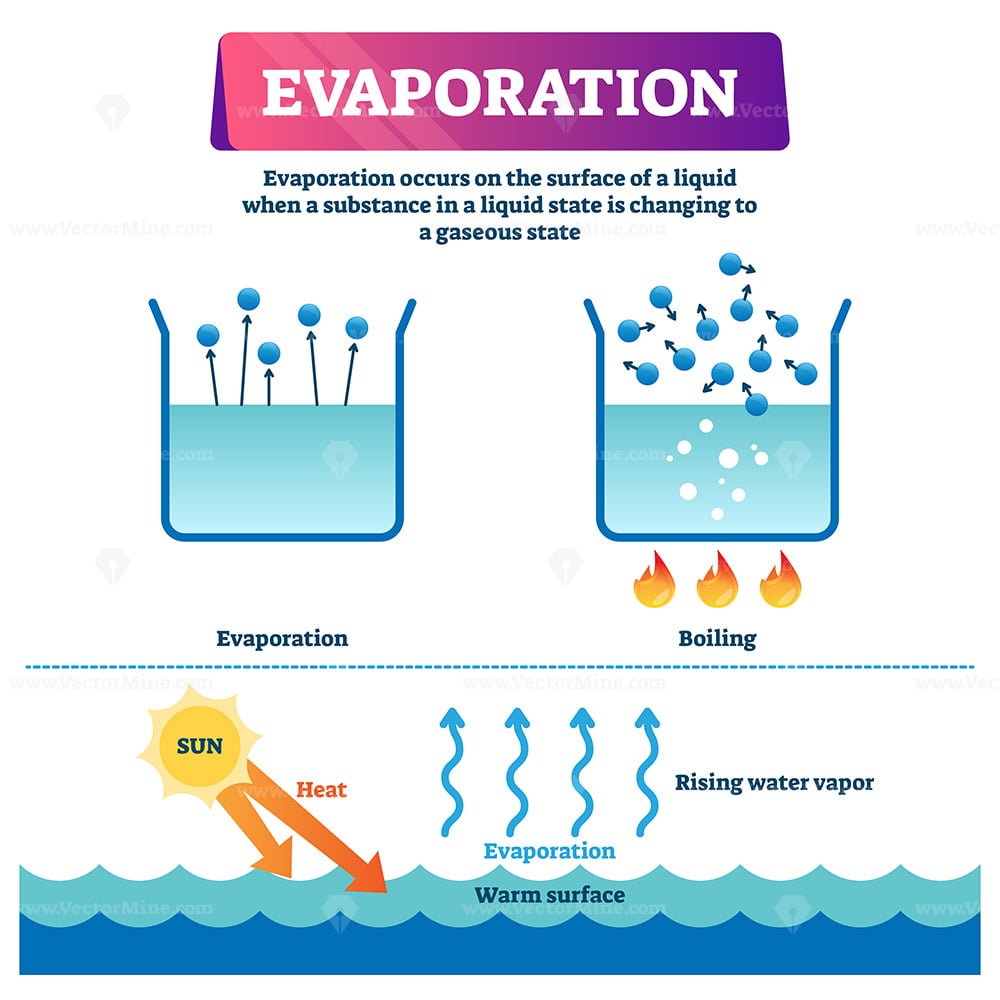
Evaporation is the natural process where the molecules of a liquid absorb the residual heat present in the. Condensation is the change of state from a gas to a liquid. As the altitude increases, the boiling point decreases.
Where the rate of change is. We can say that heating water increases the rate of evaporation because the drop of water that. Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid.
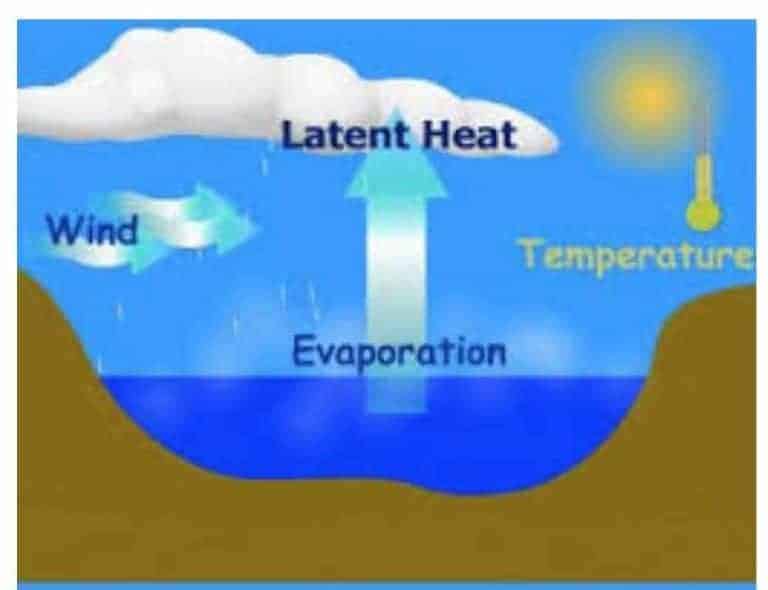
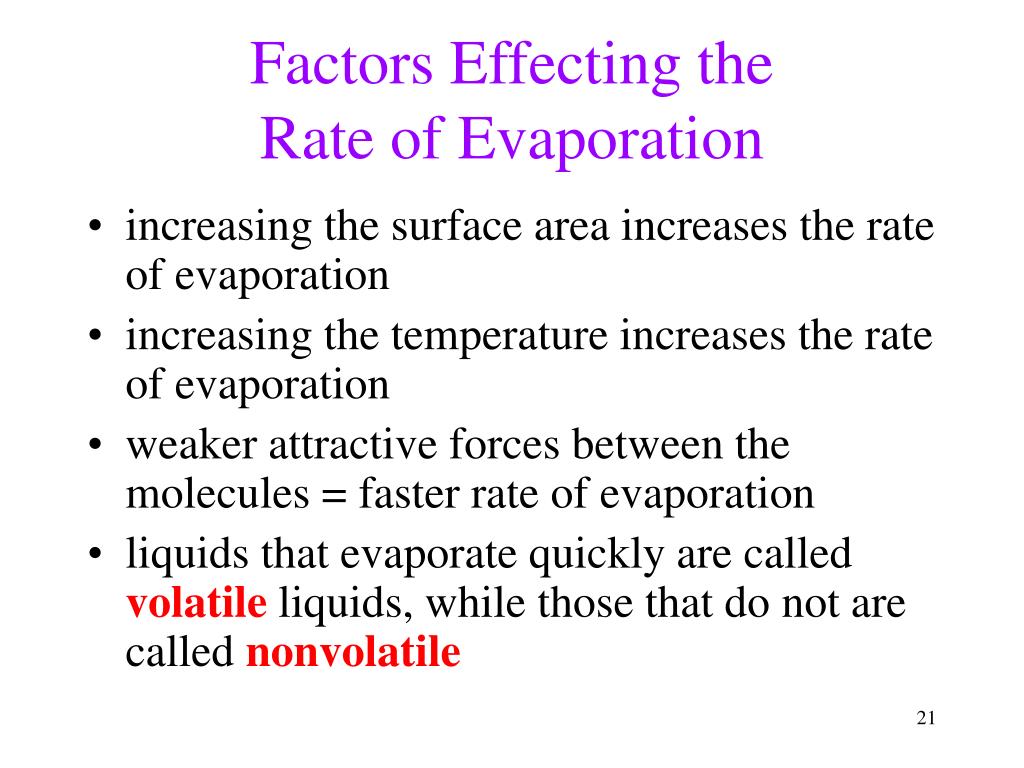
Multiply the wind speed (m/s) by 19 and add 25. Temperature of the water, humidity of the air and the surface area of the water. The evaporation rate decreased from 0.15 cm/yr in the early period to 0.17 cm/yr in the later period, and the decreasing trend accelerated slightly.
Third, rotary evaporators have a water bath that allows the solution to be warmed. To calculate the evaporation rate of a body of water, follow these steps: Vaporization is the process in which a liquid is converted to a gas.
Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. If you increase the temperature of the water the rate of evaporation. The formula for humidity ratio in saturated air is the same, except partial pressure of water vapor in moist air is replaced with saturation pressure of water vapor in.
As the altitude increases, the boiling point decreases. The rate of evaporation depends only on the surface area of the liquid and is essentially constant. This increases the rate of evaporation.
That will maximize the surface area of the liquid, maximizing the rate of evaporation. However, first it is important to understand the factors influencing the rate of.
In calculus, the rate of change refers to how a function changes between two data points. The rate of condensation depends on the number of molecules in. As the temperature increases, the rate of evaporation increases.
Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. You must have noticed that clothes dry at a much faster rate during summers than in winters when the temperatures are higher. If the temperature increases and the wind speed and humidity stay constant, then the rate of evaporation will increase since warmer air can hold more water vapor than colder air.








![broadricksec3na [licensed for use only] / Chapter 9](http://broadricksec3na.pbworks.com/f/1443158947/factors of evaporation.png)